Abstract
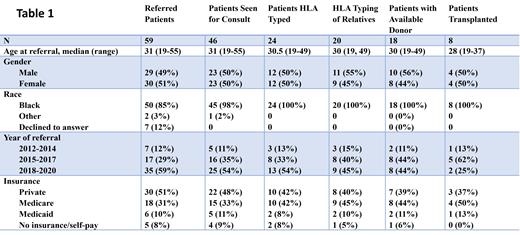
Allogeneic hematopoietic cell transplantation (alloHCT) is the only proven curative treatment for homozygous sickle cell disease (SCD) with debilitating symptoms. alloHCT may ideally be performed in children with severe manifestations of SCD before they develop irreversible organ dysfunction. However, many adult patients may also be appropriate for alloHCT. The historic lack of HLA-matched donors for this population has recently been ameliorated by the use of HLA-haploidentical donors with a T-replete graft and post-transplant cyclophosphamide (HAPLO). However, patients with SCD belong to ethnically and socio-economically underserved populations and may face several additional barriers to accessing complex and expensive curative strategies such as alloHCT. We analyzed SCD patients referred between January 2012 and April 2021 for alloHCT to our center- an adult only center with special expertise in HAPLO transplantation, located in an area with a large population of black patients. Success and barriers to progression from referral to alloHCT were assessed using our institutional database and coordinator records where they had been prospectively entered. Numbers of patients advancing from referral through stages of pre-transplant workup to alloHCT are shown in Table 1. Fifty-nine patients referred to our program for consideration of alloHCT for SCD during the period. The number of annual referrals increased during the studied period from 7 in 2012-2014 to 35 in 2018-2021. Of referred patients, 78% had an initial consult, 41% underwent high resolution HLA typing, 34% also had HLA typing of family members, 31% had an acceptable donor (HLA-identical, 8/8 matched MUD or HAPLO without donor-specific antibodies) and 14% underwent alloHCT. No significant differences were seen among the above categories with respect to age, gender, self-assigned race, year of referral and insurance status. Referrals came from a limited subset of community hematologists- with one physician each referring 9, 6, and 4 patients. Three physicians referred 3 patients, six referred 2 patients and nineteen referred 1 each. Reasons for referred patients not proceeding to alloHCT were: psychosocial issues including lack of caregiver support and history of non-compliance with treatments (n=15), insufficient disease severity by institutional criteria (n=14), failure of referred patient to attend initial consult (n=13), medical comorbidities precluding transplant (n=9), refusal of alloHCT by clinically and psycho-socially suitable patient (n=7), lack of acceptable donor despite suitable patient and search (n=4), refusal of relatives to undergo HLA-typing for an otherwise suitable patient (n=2). Patients (n=8) who underwent alloHCT a median of 253d post referral and 232d post initial consult. Transplant characteristics were: donor- HAPLO (6), MRD (1), MUD (1); graft source BM (8); intensity - NMA (4), RIC (4); median CD34 dose- 3.6x 10e6/kg (1.5-4.9); median CD3 dose 3.3x 10e7/kg (2.4-4.5); HCT-CI - <3 (2), >4(6); CMV pos patient with neg donor (3); ABO major incompatible (3). Engraftment was assessed using T-cell (CD3) and myeloid (CD33) donor chimerism. Median CD33 chimerisms were 100% on each assessment (d30, 60, 100, 180 and 360). Median CD3 chimerisms were 52%, 100%, 100%, 100%, 93% respectively. All evaluable patients achieved > 95% CD33 chimerism by d 30. Two patients (one MRD, one HAPLO) transplanted on an earlier protocol without ATG and thiotepa subsequently rejected their graft. All five HAPLO patients transplanted using the ATG/thiotepa based regimen used in BMTCTN 1507 had sustained high level engraftment (>95% CD33 chimerism) with freedom from SCD symptoms post-transplant. With a median follow-up of 39 months, three-year cumulative incidences of grade 2-4 acute GVHD, and moderate to severe NIH grade chronic GVHD were 12.5% and 0% respectively. Estimated 3 year overall survival was 85%. These data show that while most referred patients lack HLA matched donors, allo-HCT performed using HAPLO donors and RIC conditioning incorporating ATG and thiotepa with a BM graft is highly effective at eradicating SCD. However, many barriers are faced by referred patients resulting in only a small percentage of referred patients proceeding to transplant. Some of these barriers may be overcome by education of referring physicians, patients and their families
Solh: Jazz Pharmaceuticals: Consultancy; Partner Therapeutics: Research Funding; BMS: Consultancy; ADCT Therapeutics: Consultancy, Research Funding.
Preparative regimens for allogeneic transplantation typically involve off-label use of chemotherapy drugs

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal